Updated FDS Combustion and Fuel Composition Calculators
This post describes updated combustion product and fuel composition spreadsheet calculators for use with FDS (the Fire Dynamics Simulator from NIST). The first calculates combustion products given fuel composition and CO and Soot yields. The second does the inverse calculation for fuel composition given the product yields. We first provide detailed descriptions of the solution methods and then update and correct errors in a previous post that calculated fuel composition for the New Zealand C/VM2 - Verification Method Fire. Both calculators are available on the PyroSim Resources web page.
Simple Chemistry Model
For most applications, the FDS "simple chemistry" model is appropriate. Although it is called the "simple chemistry" model (and is in fact simple in the sense that combustion is modeled using only three species - fuel, air, and products), for me (and I expect for many engineers) it takes some review to be comfortable with the calculations.
Chapter 12 of the FDS User Guide discusses combustion. The simple simple chemistry combustion model, considers a single fuel species that is composed of C, H, O, and N that reacts with oxygen (air) in one mixing-controlled step to form the product species consisting of H2O, CO2, Soot, N2, and CO. The user specifies the chemical formula of the fuel along with the yields of CO and Soot, and the volume fraction of hydrogen in the soot, XH. FDS uses that information to calculate the stoichiometric coefficients as follows:
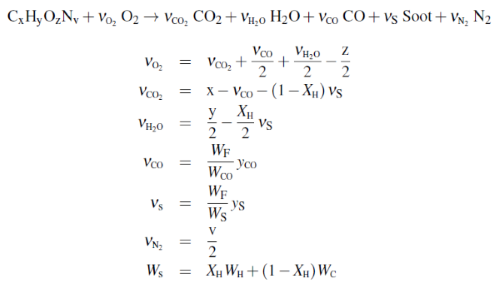
Mixing-controlled means that the combustion reaction occurs immediately when fuel and air are available in a cell.
Combustion Product Calculator
Let us start with the combustion calculator and to make sure we understand these equations, we will go through a complete derivation. The key concept is that we balance the C, H, O, and N atoms in the fuel and air with those in the products. Here are the details.
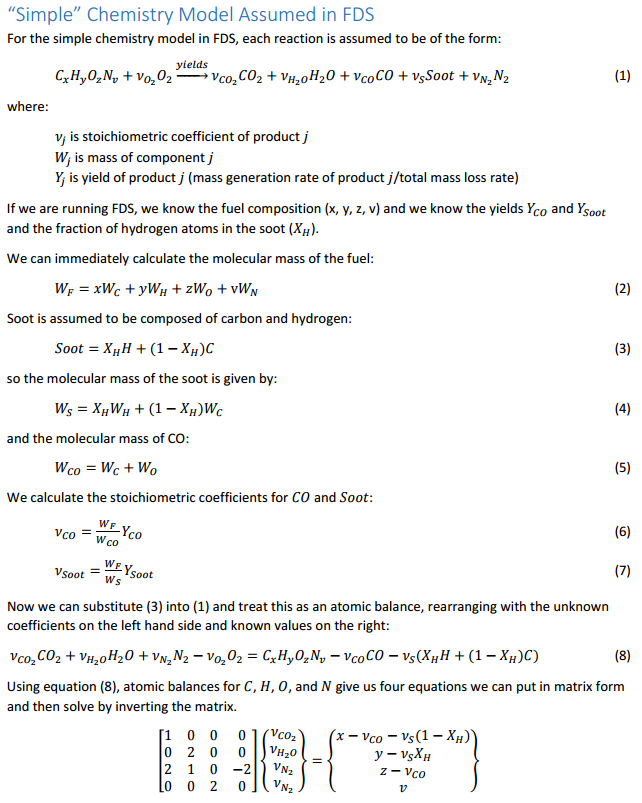
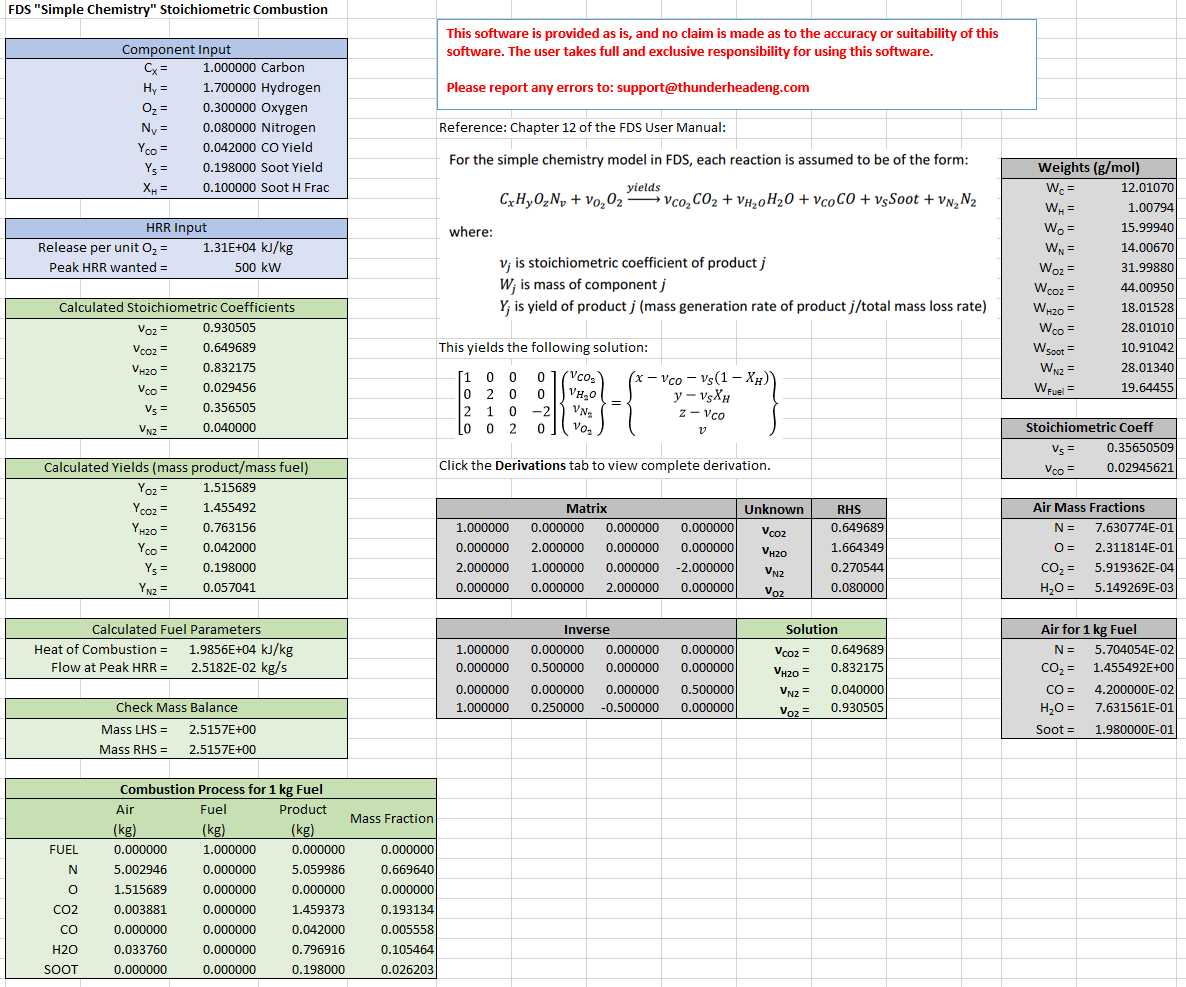
The above derivation provides a set of simultaneous equations, where each row in the matrix results from the balance for a single element (C, H, O, and N). We can solve this in a spreadsheet. The solution below is for the default polyurethane reaction in PyroSim, note that the input corresponds to a fuel , with a CO yield of 0.042, a Soot yield of 0.198, and a fraction of H in Soot of 0.1. The calculated stoichiometric coefficients are , , , and .
During a solution, FDS writes a *.out file that contains information on the reaction. I have copied the part of that output that shows that the mass fractions in the product species in the spreadsheet match the FDS calculations (compare to the last column in the Combustion Process for 1 kg Fuel table above).
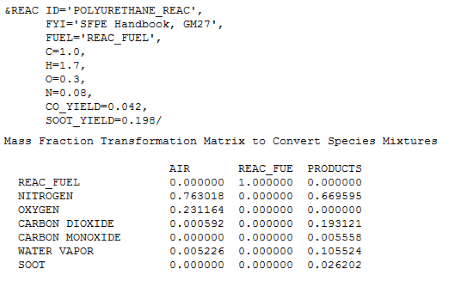
An earlier version of our combustion calculator just used the FDS solution in the User Manual (see above) and that gives the same result as the present calculation. So we can feel comfortable that we are correct in our derivation and implementation.
Fuel Composition Calculator
This is essentially the inverse of the combustion calculator. For this case, we know the products and want to calculate the fuel composition that corresponds to these products. The derivations are similar to those described above. At the end of the derivation, we have a matrix that allows us to solve for the unknown fuel composition and oxygen (from the air) stoichiometric coefficient.
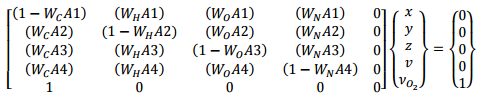
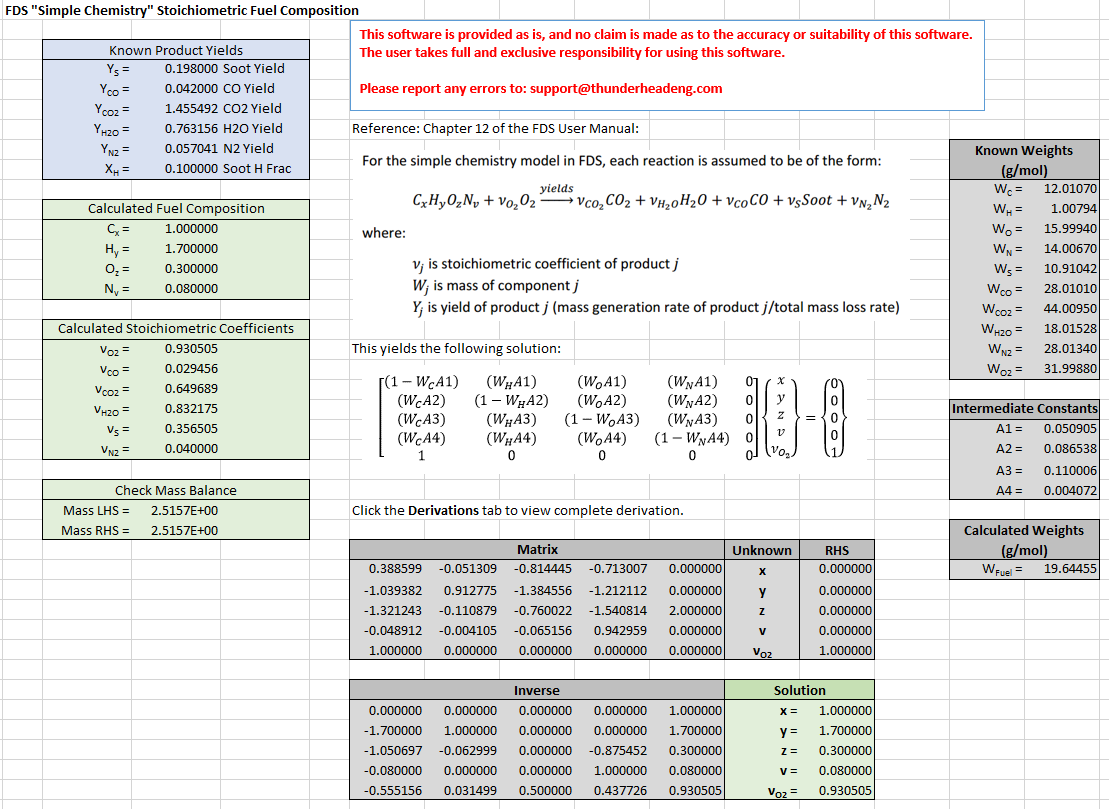
We can demonstrate this using the output of the combustion product calculator ( Soot yield of 0.198, CO yield of 0.042, CO2 yield 1.455492, H2O yield 0.763156, N2 yield 0.057041, and fraction of H in Soot of 0.1) as input to this calculation. We obtain the original fuel composition fuel and calculated stiochiometric coefficient for
So now we can go both ways, if we know the fuel we can calculate the products and, if we know the products, we can calculate the fuel.
NZ Fuel Composition
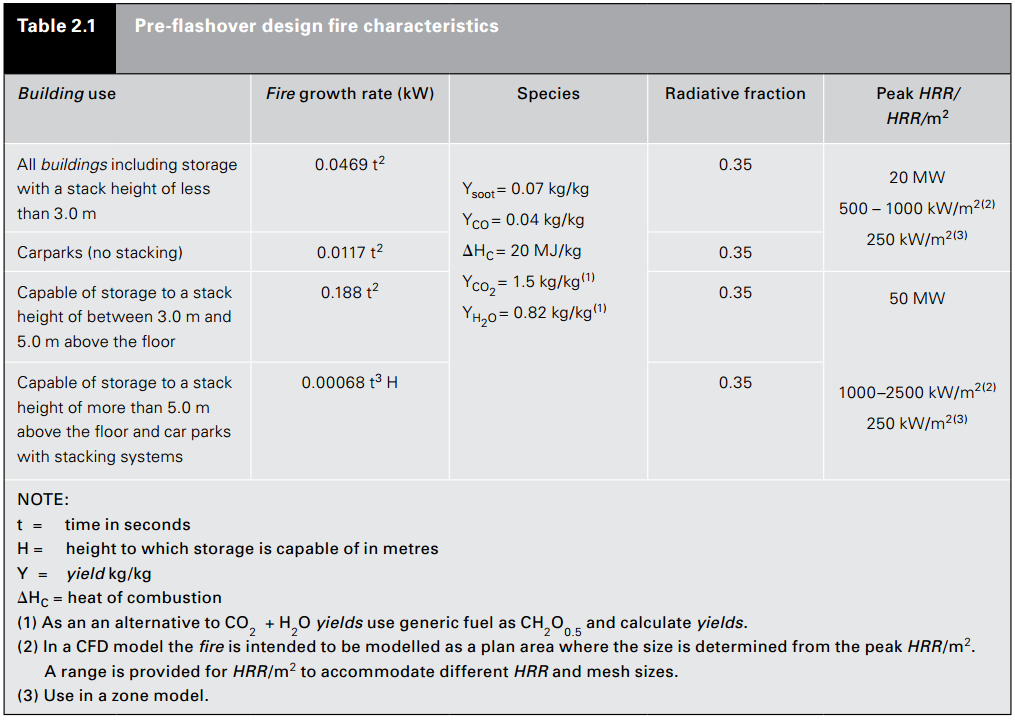
Finally, we return to the calculation that started this discussion. My goal was to calculate the fuel composition that corresponded to the New Zealand building code, C/VM2 - Verification Method: Framework for Fire Safety Design. The table below gives the design fire characteristics. Note that the table defines the yields, however Note (1) does provide an alternate generic fuel for calculations. My first mistake in the previous post was to ignore Note (1). But, since we now have a fuel composition calculator, let us use the NZ products to calculate a fuel composition.
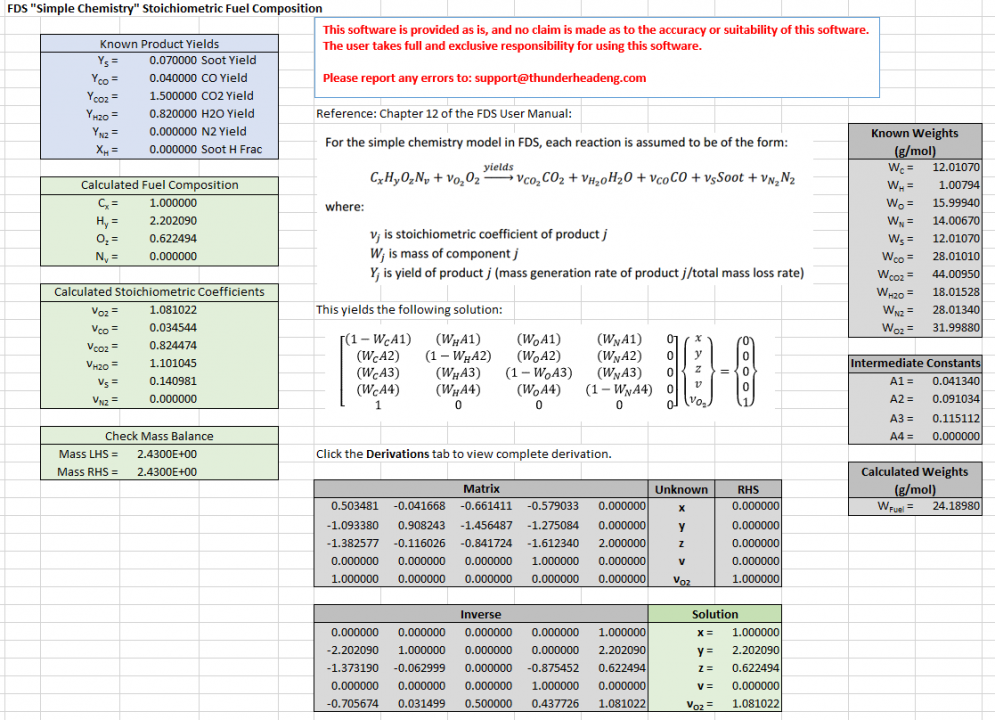
We input the yields defined in the table ( Soot yield of 0.07, CO yield of 0.04, CO2 yield 1.5, H2O yield 0.82) and obtain a fuel composition of . The heat of combustion is (assuming an energy release per unit mass of oxygen, EPUMO2, of ). Both the fuel composition and heat of combustion are reasonably close to the alternate fuel and alternate 20 MJ/kg heat of combustion. In addition, conversations with practicing engineers indicate that a fuel of is reasonable (see the Acknowledgements below).
Conclusions
We have derived in detail the combustion calculation and inverse fuel composition calculation that corresponds to the "simple chemistry" model in FDS. The intent is to help the reader more completely understand the stoichiometric calculations in FDS.
A key comment by Jason Floyd is that "Essentially all common combustibles sit near a EPUMO2 of 13 MJ/kg." I had violated this in my original calculations.
Acknowledgements
I would like the thank Jason Floyd at Jensen Hughes, Christofer Wickmark at Holmes Fire, and Antony Walker at Holmes Fire for graciously pointing out that I had made an error in my first calculations. Further discussions with them helped me prepare this updated post. These engineers obviously know what they are doing.
References
C/VM2, Verification Method: Framework for Fire Safety Design, Amendment 4. 2014. The Ministry of Business, Innovation and Employment, PO Box 10-729, Wellington.
McGrattan, Kevin, et al. 2014. Fire Dynamics Simulator User's Guide (Sixth Edition). Gaithersburg, Maryland, USA, July 2014. NIST Special Publication 1019-6.
Comments or Questions
This post was written by Daniel Swenson. For comments or questions, send email to support@thunderheadeng.com.